
四種基本甘斯的製作方法
凱史基金會教育團隊負責
2018年12月
摘錄自等離子體月刊第七期
這篇文章提供讀者簡易遵循一步步的指引,如何做出四種基本甘斯,這四種甘斯以不同的方式與我們的身體連結。相關主題的其他資訊,可參考KF Wiki1和KF SSI YouTube頻道2。也一併介紹在生產這些甘斯背後的基本理論,每一種甘斯與人體相關的部份。
先退後一步,讓我們首先想一下甘斯到底是什麼。甘斯GANS是氣體在奈米的固體狀態的縮寫,如凱史先生所解釋的。凱史在他的第二本書,『這種物質的甘斯條件是在周圍溫度與壓力下,因為原子內部的磁引力場強,原子的物理外觀改變,以及氣體改變的原子結構變成固態的緊密配置。同樣氣體的原子變成也表現得像固體,但具有全新的性質和特性,是除了原子處於另外三種早先已知的狀態之外,從不被知道的。(亦即氣體,液體和固體以外。)』(Keshe,2011)。
有很多方法可以生產甘斯群。一般是用兩塊(或兩塊以上) 的金屬板子或金屬線,其中一個經過奈米塗層。圖一概述做四種甘斯會用到哪些金屬。也有另外的方法,比方,要做維他命甘斯、食物甘斯、或其他物質的甘斯、可以透過氫氧化鈉的使用或二氧化碳甘斯的等離子體水去生成
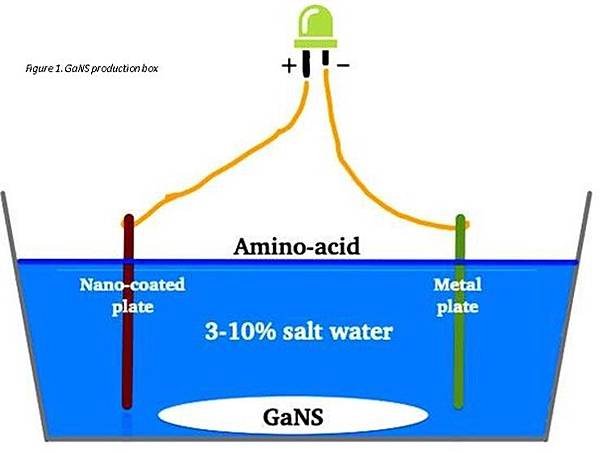
甘斯生成捕捉盒類似吸收食物或吸進空氣的過程,不管是通過肺壁,或通過腸壁。但都是產生一個特定的條件,以便吸引氧(肺部),以及其他物質(腸道)的場域。這樣人體(甘斯生成盒)產生一個『磁鐵』的條件,吸引特定強度的場域 (較弱,因此決定流動的方向)。被吸引的場域在鹽水的環境慢下來轉換成既定元素的物質狀態。
做任何甘斯所需要的材料清單如下:
- 塑料盒(可用奈米塗層過的,可加快生產速度)
- 一個金屬板或線圈
- 一塊奈米塗層過的金屬板或線圈
- 純海鹽以及蒸餾水
- 銅線用於圍成迴路
- 綠色LED
- 兩個鱷魚夾(如果你不要鑽孔,就可選這個)
- 奈米塗層過的塑膠湯匙,用來撈生成的氨基酸
- 兩個塑膠容器或玻璃容器用來裝生成的氨基酸
- 針筒收集甘斯或去除多餘的水
第一張圖表. 生產四種基本甘斯所用到的金屬。
製作程序
步驟一
根據你要做的甘斯(參考第一張表),其中一塊金屬板(或線)奈米塗層過。也可參
照教學影片3。在此過程之前,如果使用的是金屬板,不是線圈,要在兩塊板子的角落鑽孔(這樣就不需使用鱷魚夾)。如果沒有鑽孔工具,就用鱷魚夾代替。
步驟二
將兩塊板子懸在甘斯生成捕捉盒中,彼此相對,相隔幾公分,如圖一所示。板子或線圈不能碰到容器底部,而要固定懸在離底1-2英寸上方處(2.5~5公分)。 如果碰到盒子底部,會減慢或阻礙甘斯的生成。
步驟三
用銅線連接兩塊板子,藉由鑽孔或使用鱷魚夾。還有替代方式,可以在迴路上放置綠色LED。在迴路使用LED燈有助於在板子之間產生能量流。
每個甘斯生產都一樣,LED線較長的一邊是陽極,另一邊陰極。連到銅線與金屬板或線連結。
步驟四
填滿甘斯生成捕捉盒約3.5-10%的海鹽,以及蒸餾水溶液 (確定鹽沒有被任何礦污染過)。將盒子與其他盒子和反應器隔開,自行放置幾個星期。每種甘斯生成所需時間取決於幾個因素,比方生成甘斯的種類,金屬板彼此相隔的距離,奈米塗層的品質,金屬的純度以及其他。
步驟五
當觀察到盒子底部有甘斯生成物,或金屬板有鹽度黏附或脫落磨損,就是收成甘斯的時候。把金屬板拿開放置一旁,先收集浮在水錶面的氨基酸,存放在乾淨的玻璃或塑膠容器裡,用蒸餾水加滿,不要讓它乾掉。
步驟六
不管是用針筒收集盒子底部的甘斯沉積物,或是小心移走多餘的水把沉積物,倒入分開的玻璃容器或塑膠容器儲存。清洗甘斯降低鹽度是個選擇,看你要把它應用在哪裡。詳細內容與圖片可參考KF wiki.4

#小撇步
- 確定在每次甘斯生成之前,你上一次用苛鹼做奈米塗層的金屬板有洗乾淨
- 小心處理奈米材料。不要刮傷它,動作放輕,不要用疊的。
- 用苛鹼(氫氧化鈉)做奈米塗層,比火烤更耐久。
- 確定你用的鹽沒有雜質。
- 生產甘斯不僅可考慮用海鹽或食鹽,還可用其他鹽,比方鎂或鉀。
- 甘斯生成捕捉盒先做奈米塗層效果更好,或只要拿前一次做同樣甘斯的盒子來使用,因為在生產過程盒壁已經有奈米的塗層。
- 因為甘斯生成捕捉盒的水分會蒸發,分解成甘斯,有些鹽的結晶體會沉澱在底部,降低水的鹽度。如果需要可再加入(蒸餾)水,以及如果需要再加入一些鹽。
- 每個甘斯生成捕捉盒要相隔至少3-5公尺,才不會彼此在生成過程互相干擾,以達到預期的效果。
- 不要用太薄的金屬板/線圈,因為不是在奈米塗層就是在甘斯生成過程容易折斷。
- 生產出來的每種甘斯在強度上有所不同,把它當成同一個元素的同位素。所以,為了涵蓋廣泛的場域頻譜,比方把最近剛做的CO2 甘斯,加到之前做好的CO2甘斯罐。經過一段時,你的CO2甘斯會有很多不同風情,在任何應用領域會更有效。
#如何作用
讓我們回想生產CO2甘斯用到鋅與奈米銅。銅的原子量剛好超過63.5(銅的同位素69%的是Cu63,31%幾乎是Cu65),鋅是65(超過49%是Zn64,其他同位素是66-68)5。在奈米塗層的過程,任何金屬會損失原子量約5%。因此,銅減少到約59的原子量。兩個板(65和59)之間的差異是6,是原子碳的數量(內核的質子數)。所以CO2生產捕捉盒,在兩塊板子之間,產生一個強度6的等離子體場域,像是一塊磁鐵,吸引盒子周遭大氣裡的碳。
在等離子體,較強的餵養較弱的以找到平衡,因此捕捉盒所產生的場域較弱的一部分。在鹽水溶液中,碳在有氧的等離子體下氧化,以CO2甘斯的形式沉入底部。
氧化鋅甘斯是生產CO2甘斯的副產物。奈米銅板產生一個條件,從鋅板把鋅吸過來,氧化成氧化鋅。結果CO2生產捕捉盒生產出CO2與氧化鋅的混和甘斯。兩者之間的比例,看作的人如何設定。 更多細節可上KF wiki找到。
CH3甘斯以類似方式生成,是兩階段的過程。首先,奈米銅(59)與鍍鐵鋅層相互作用。碳的磁鐵作用吸引這個元素,結果產生二氧化碳甘斯沉澱在盒底。 其次,奈米銅板的場域與鐵相互作用(原子量接近56,幾乎是Fe56的92%)並為氚產生了『磁鐵』。從大氣吸來的氚與鹽水環境存在的碳場域結合,以CH3甘斯的形式沉澱到捕捉盒底。
氧化銅與氧化鋅的生產用到同樣的金屬。氧化銅是銅與奈米銅,氧化鋅是鋅與奈米鋅。奈米塗層金屬板,把另一塊板子的元素吸引或拉出來,在等離子體水的環境氧化,以甘斯的形式沉到容器底部。甘斯群在生產它的母環境獲得相似性。
因此,CO2 甘斯對銅對鋅以及對鈉(因為NaCl存在水中)具有親和力。除此,不管在奈米塗層過程用了什麼材料,所產生的甘斯也會對它們有親和力,例如,在奈米塗層過程用到氫氧化鎂Mg(OH)2。每個產出的甘斯強度都不同,因為它與產生它的元素和環境有關。換句話說,這些是知識尋求者在不同內容下聽到的共同點。
#觀察
在做甘斯過程會觀察到以下情況。首先,氧氣泡泡出現在奈米金屬板/或線圈。 幾天後(或幾週後,取決於設置),可注意到捕捉盒底有沉澱物以及在水的表層出現薄薄的氨基酸。通常,金屬板露出水面上的會有鹽結晶以及沉積物。
#用法
甘斯與氨基酸的生產,是這星球生命開始的方式。全都始於海洋的鹽水環境以及周圍的物質狀態與甘斯狀態。在甘斯生產過程中,兩塊金屬板在鹽水環境創造條件某些元素,像是碳,鋅,銅,氧或氫。這些都是這個星球生命賴以維生的基本元素。
表2顯示這四種基本甘斯與人類的關聯。甘斯群是等離子體技術的基礎,應用在於很多領域,像是食品、農業、健康、能源、消除污染,太空旅行等以及其他。從不同甘斯捕捉盒收集來的氨基酸有不同強度,因為它們會被介於兩塊金屬板之間不同強度的『等離子體磁鐵』給吸引。
甘斯也可以底部沉澱物收集到的水,與蒸餾水混和做出液體的等離子體水。應用在健康與農業時,會在等離子體水再多加胺基酸,以強化與生物體的連結性,因為生物體正是奠基於氨基酸。
致謝
這篇文章的內容基取自凱史先生所傳授的知識與發現。以及其他知識尋求者,多年來以影片以及維基新增的形式記錄下來。
Method to produce the four basic GANSes
by KF SSI Education Team
December 2018
This paper provides the reader with an easy to follow, step-by-step guide on how to produce the four basic GANSes, which are in various ways connected to our bodies. Other resources relating to this topic exist on the KF Wiki1 and the KF SSI YouTube channel2. The basic theory behind the production procedure is explained together with the affinity of each of these GANSes to the particular aspects of the human (or any other animal’s) body.
Taking a step back, let’s first consider what GANS actually is. GANS is the abbreviation for Gas in Nano-state of Solid and as explained by M.T. Keshe in his second book, "This condition of GANS of matter comes about at ambient temperature and pressure when due to internal Gravitational and Magnetic fields’ strength of the atom the atom’s physical appearance changes and the atomic structure of the gas changes to a compact configuration of the solid. The atom of the same gas becomes and behaves like a solid but with totally new properties and characteristics which were never known when the atom was in its other three initial known states (gas, liquid and solid)." (Keshe, 2011).
There are many methods through which one can produce GANSes. Traditionally, this is achieved through the use of two (or more) metal plates or coils, one of which is Nano-coated. Table 1 gives an overview of which metals are used in the production of the four GANSes. Other methods exist, for example, GANS of vitamins, food and other substances can be prepared through the use of caustic soda or CO2 GANS and plasma water.
The process in a GaNS production box is similar to the process of absorption of food or air – no matter crosses the wall of the lungs or intestine, but a certain condition is created to attract the field of the elements of Oxygen (lungs) and other matter consumed (intestine). In this way human body (and the GANS production box) creates a condition of a ‘magnet’, which attracts fields of a specific strength (being the weaker and hence dictating the direction of the flow). The attracted fields, in the salty water environment, slow down and transform into the matter state of a given element. The complete list of materials required for the production of any of these four GANSes is as follows:
Plastic box (optionally Nano-coated for faster production)
One metal plate or coil
One Nano-coated metal plate or coil
Pure sea salt and distilled water
Copper wire for short-circuiting of the metals
Green LED
Two alligator clips (optional if plates are not drilled)
Nano-coated plastic spoon to harvest amino- acids
2 x Plastic or glass containers to store amino- acids and GANS
Syringe to collect the GANS or to remove excess water
Table 1. Metals used in the production of the four basic GANSes.
Production procedure
Step 1)
Depending on which GANS one wants to produce (as per Table 1), Nano-coat one of the plates/coils following any of the available video or text guides3. Prior to this process, if using metal plates instead of coils, drill a hole in a corner of both plates (this eliminates the need for the use of alligator clips). If no drill available, alligator clips can be used.
Step 2)
Hang the two plates in the production box, opposite each other at a distance of several centimeters - as per Fig. 1. Plates or coils should not touch the bottom of the container but instead should be fixed 1-2’’ above its bottom. If they sit on the bottom of the box, this will slow down or prevent GANS from being produced.
Step 3)
Connect the two plates via a copper wire either by going through the drilled holes or using alligator clips. Optionally when connecting the plates, one can place a green LED in this circuit. The use of an LED light in the circuit helps to create the flow of energy between the plates. As with every GANS production, connect the anode (longer piece of wire = positive pole) of the LED with the copper wire that leads to the Nano-coated metal plate/coil, and the cathode (negative pole) with the copper wire that leads to the metal plate/coil (Fig. 1).
Step 4)
Fill up the GANS production box with a 3.5-10% sea salt, distilled water solution (make sure the salt is not contaminated by any minerals). Place the box away from other boxes and reactors and leave alone for couple of weeks. The time needed to produce each of these GANSes depends on a number of factors, such as type of GANS produced, materials available in the proximity of the production box, water salinity, distance between the plates, quality of the Nano-coating, purity of
metals and other. Step 5)
Step 5)
When one observes the deposit of the GANS at the bottom of the box, or the plates become too salty or worn out, it is time to harvest the GANS. Set the plates aside and with a Nano-coated plastic spoon, collect the amino acid which floats on the water’s surface. Store it in a clean glass or plastic container and top it up with distilled water not to dry out.
Step 6)
Either use a syringe to collect the GANS sediment from the bottom of the production box or carefully remove the excess water with it and pour the sediment into a separate glass or plastic container to store it. Washing of the GANS to reduce salinity is optional and depends on the application area.
For more information and pictures, detailed guide on the production of the four basic GANSes is available on the KF wiki.4
Useful Tips
Make sure you wash your Nano-plate of any caustic soda residue, before using it in the GANS production.
Handle Nano-materials with care. Do not scratch it, be gentle with it and never stack it up.
Use caustic soda Nano-coating method which results in more permanent Nano-layers than fire-coating.
Make sure the salt you are using has no impurities.
You can produce GANSes not only through the use of sea or table salt (NaCl) but also using other salts such as Magnesium or Potassium.
Nano-coat your GANS production box for better results or simply reuse one from a previous GANS production of the same GANS as during the production process walls of the container get Nano-coated.
As water from your GANS production box evaporates and decomposes into GANS, some of the salt crystals can deposit at the bottom, which reduces water salinity. Top up your boxes with distilled water and if needed add some salt into the distilled water used for topping up.
Keep you GANS production boxes at least 3-5 meters apart in order not to influence the process in each box and to achieve the expected results.
Use plates/coils which are not too thin as they can break either during the Nano-coating or GANS production processes.
Every GANS produced is different in strength think of it as isotopes of the same element. Therefore, in order to cover a wide spectrum of fields, add your e.g. freshly produced CO2 GANS into the jar/container with previously produced CO2 GANS. Over time, your CO2 GANS has many different ‘flavors’ to it and can be more effective in any application area.
How does it work
Let’s consider the CO2 GANS production in which a Zinc and a Nano-coated copper plates are used. Atomic weight of Copper is just over 63.5 (69% of the copper isotopes is Cu63 and almost 31% is Cu65) and Zinc is 65 (just over 49% is Zn64, other isotopes are in the range of 66-68)5. In the Nano-coating process, any metal loses around 5% of its atomic weight. Therefore, Copper reduces to around 59 atomic weight. The difference between the two plates (65 and 59) is 6 and it is the atomic number (number of protons in the nucleus) of Carbon. Therefore, the CO2 production box creates a plasma field between the two plates of the strength 6, which like a magnet, attracts Carbon present in the atmosphere around the box. In plasma, stronger feeds the weaker to find balance, and hence the field created in the box is the weaker part. In the salty water solution, Carbon oxidizes in the presence of the plasma of Oxygen and deposits as a CO2 GANS at the bottom.
The side effect of the CO2 GANS production is the presence of ZnO GANS. The Nano-coated copper plate creates a condition for attraction of Zinc from the Zinc plate, which in turn oxidizes to become the ZnO. As a result, the CO2 box produces a mixture of CO2 and ZnO GANS, where the ratio of the two depends on the setup. More details can be found on the KF wiki page on the CO2 GANS production6.
GANS of CH3 is produced in a similar way and is a 2-stage process. Firstly, the Nano-copper (59) interacts to find balance with the layer of Zinc of the galvanized Iron. This ‘magnet’ for Carbon attracts this element and as a consequence the CO2 GANS gets produced and deposits at the bottom of the box. Secondly, the fields of the Nano- coated copper plate interact with Iron (atomic weight close to 56, with almost 92% of Fe56) and create a ‘magnet’ for H3. Tritium attracted from the atmosphere binds with Carbon fields already present in the salty water environment and deposits as the CH3 GANS at the bottom of the production box.
CuO and ZnO GANS productions use the same metals to produce it. For CuO it is Copper and Nano-copper, for ZnO it is Zinc and Nano-zinc. The Nano-coated metal plate attracts/pulls elements out from the other plate, which then oxidize in the plasmatic water environment and deposit at the bottom of the container in the form of GANS. GANSes acquire the affinity towards the environment which produced it.
Therefore, the CO2 GANS has affinity towards Copper and Zinc, as well as towards Sodium (as NaCl is present in the water). Moreover, whatever materials are used in the Nanocoating process, the produced GANS also has affinity towards them e.g. using Magnesium hydroxide Mg(OH)2 in the Nano-coating process. Therefore, each of the produced GANSes differs in strength as it is affined to the elements and environment which produced it. In other words, these are the common denominators Knowledge Seekers hear so much about in various context.
Observations
During the GANS production one observes the following. Firstly, oxygen bubbles appear on the Nano-metal plate/coil. After couple of days (or weeks, depending on the setup) one can notice a sediment deposition at the bottom of the production box and a thin layer of amino-acid building on the water’s surface. Often, salt crystals build up and deposit on the part of the Nano-plate which is above the water’s surface.
Usage
The GANS and amino-acid production is the way life started on this planet. It all started in the salty water environment of the Oceans and surrounding matter and GANS state. During GANS production process, the two metal plates in a salty water environment create a condition for attraction of certain elements e.g. Carbon, Zinc, Copper, Oxygen, or Hydrogen. These are the basic elements, which life on this planet is based on.
Table 2 shows the connection of these four basic GANSes to the human being. GANSes are the ba-sis of the Plasma Technology and are applicable in many areas, such as Food and Agriculture, Health, Energy, Decontamination, Space Travel and other. Amino-acids collected from different GANS production boxes are of different strength in respect to each other, as they get attracted by different strength `plasmatic magnets` created between two metal plates.
GANS is also used to prepare the liquid plasma by mixing it with distilled water and collecting the water above the sediment. For applications in Health and Agriculture, amino-acid is added in the process of preparing the liquid plasma to strengthen its connection to living organisms, which are all based on amino-acids.
Acknowledgements
The content presented in this publication is based on the knowledge and discoveries of M.T. Keshe and other Knowledge Seekers who over the course of the years documented it in the form of videos and wiki articles.
Supplementary material
An extensive guide on how to produce these four basic GANSes is available on the KF Wiki. These guides contain links to YouTube videos and other handy resources. For more details please visit: https://en.kfwiki.org/wiki/Category:Nano- Coating_and_the_Production_of_GANS
References
1https://kfwiki.org/ 2https://www.youtube.com/channel/UCtQzN7XLiLvTpyUnQAa1mRw
3https://en.kfwiki.org/wiki/Category:Nano-Coating_and_the_Production_of_GANS#Nano-
Coating_Using_NaOH
4https://en.kfwiki.org/wiki/Category:Nano-
Coating_and_the_Production_of_GANS#Different_Types_of_GANSes
5https://ptable.com/ 6https://en.kfwiki.org/wiki/CO2_GANS
7https://store.keshefoundation.org/store/product/GANS_Reserve_Bottle/
Keshe, M. (2011). The Structure Of The Light. Stichting the Keshe Foundation.
PLASMA SCIENTIFIC JOURNAL


 留言列表
留言列表

